Medical research
In order to start clinical trials with its treatment, Nanobacterie carries out research in the following areas:
1. The development of an injectable suspension of magnetosomes
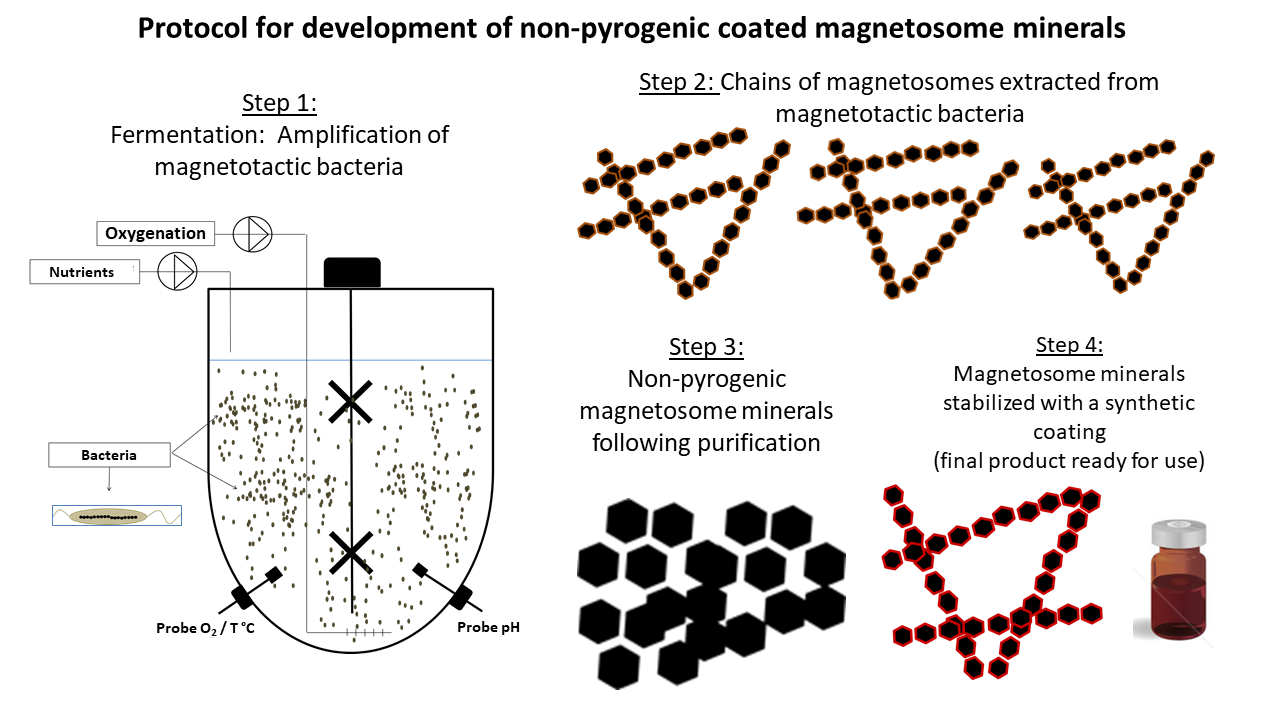
The development of the injectable suspension of magnetosomes involves four steps:
- Growth of magnetotactic bacteria in a fermenter (step 1);
- Magnetosome extraction from magnetotactic bacteria (step 2);
- Purification of magnetosomes to obtain magnetosome minerals composed essentially of ferrimagnetic iron oxide (step 3);
- Coating of these minerals with a synthetic material to make them stable in suspension and injectable (step 4).
The manufacturing process is optimized to achieve the maximum production yield of magnetosomes, a purification level that results in the removal of most of the immunogenic organic material surrounding the magnetosome minerals, and a stabilizing coating that enables injection of the magnetosome minerals.

The figure below details the four steps involved in the manufacture of the magnetosome suspension.

2. Biocompatibility of coated magnetosome minerals
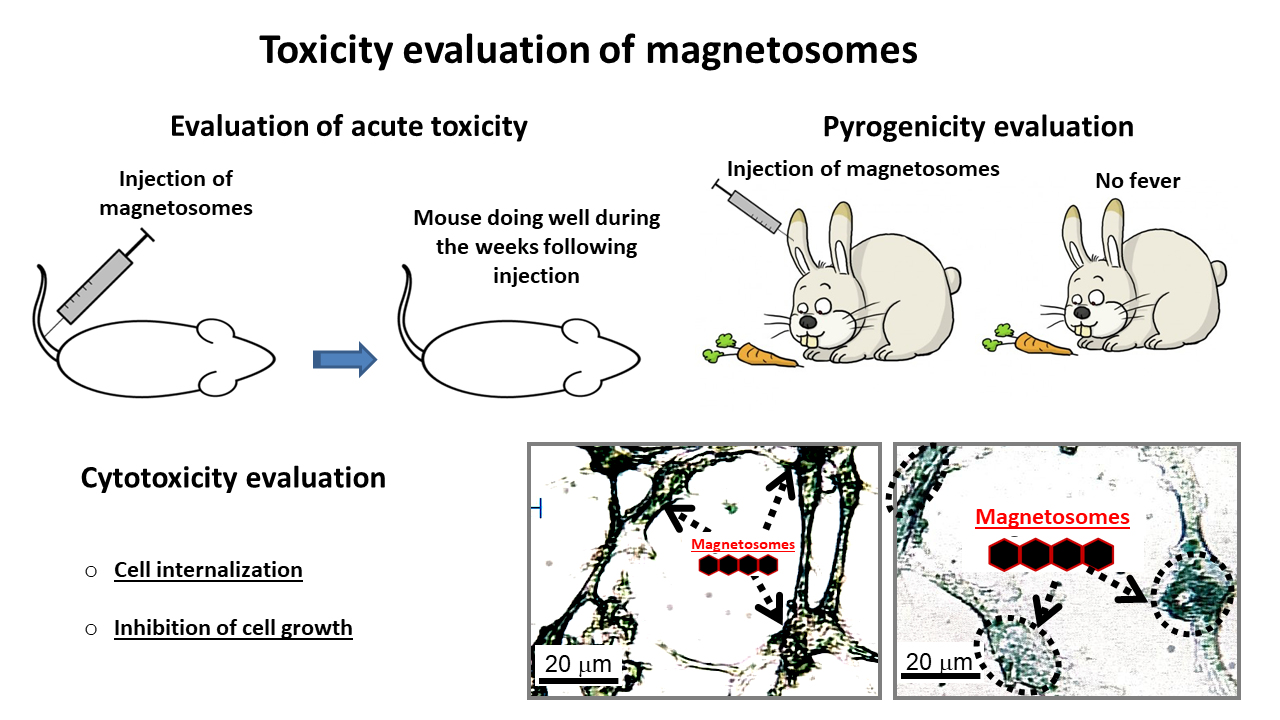
Nanobacterie carries out tests in a specialized and accredited laboratory to verify that the suspension of magnetosomes that we develop is non-toxic.
The figure below summarizes some of the tests we carry out to evaluate acute toxicity, pyrogenicity, and cytotoxicity of the magnetosomes.
3. Efficacy of coated magnetosome minerals
Nanobacterie has studied the efficacy of our treatment in murine models of mammary tumors and glioblastomas. Tumor models used include:
- Subcutaneous MDA-MB231 breast cancer tumors and subcutaneous glioblastomas GL-261;
- Intracranial glioblastomas U87-Luc.
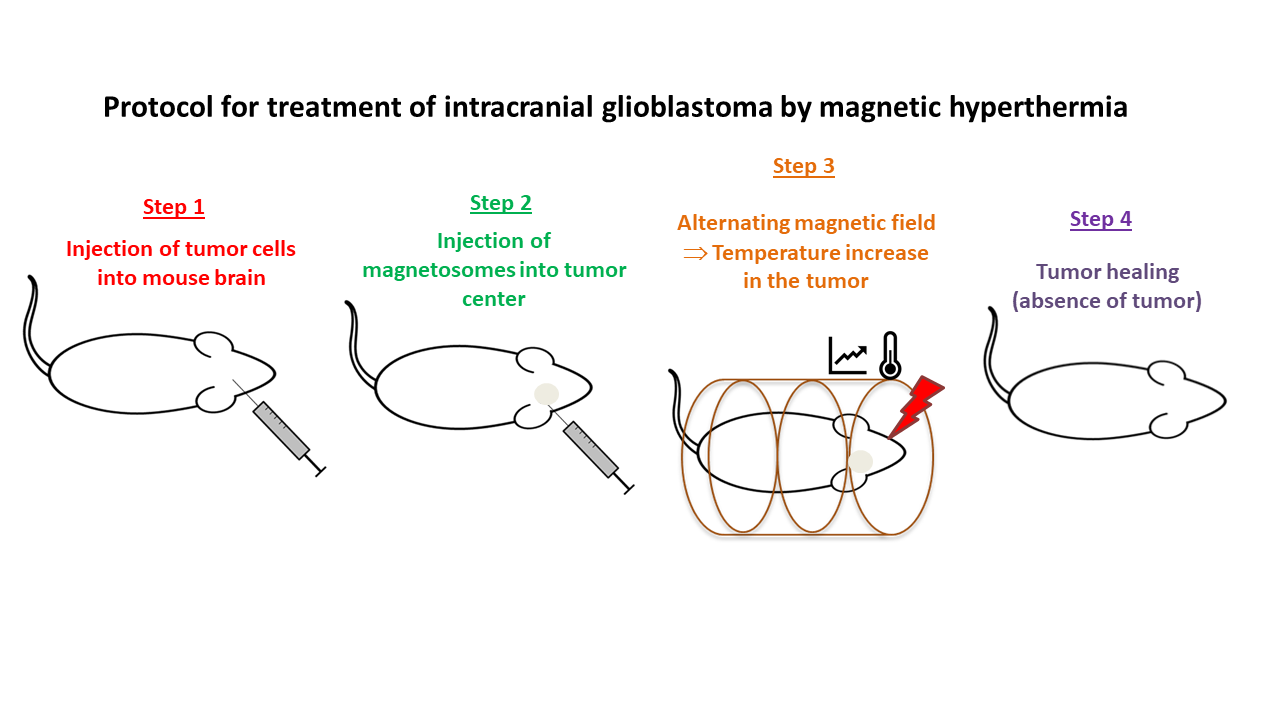
The treatment protocol involves four successive stages:
- Injecting tumor cells in mice to grow the tumor (step 1);
- After 15 days, injecting the magnetosome minerals into the center of the tumors (step 2);
- Exposing mice to several sessions of application of an alternating magnetic field, the frequency and strength of which are adjusted to reach the desired hyperthermia temperature (typically between 41 and 50°C (step 3);
- Monitoring the growth of the tumor and checking that the tumor has disappeared several weeks after starting treatment (step 4).
The figure below shows the different steps involved in the treatment of subcutaneous tumors (MDA-MB231 and GL-261):

The figure below presents the different steps involved in the treatment of intracranial glioblastomas (U87-Luc):